RIMTrack – New Age Regulatory Information Management (RIM) tracking for the Life Sciences & Healthcare Organizations
With ever-changing national regulations, data standards, and complexities around preparing for regulatory submissions, regulatory affairs organizations in Healthcare and Life Sciences industry are convinced and driven towards smarter processes and intelligent systems to optimize costs, maximize accuracy and minimize timelines for the submission.
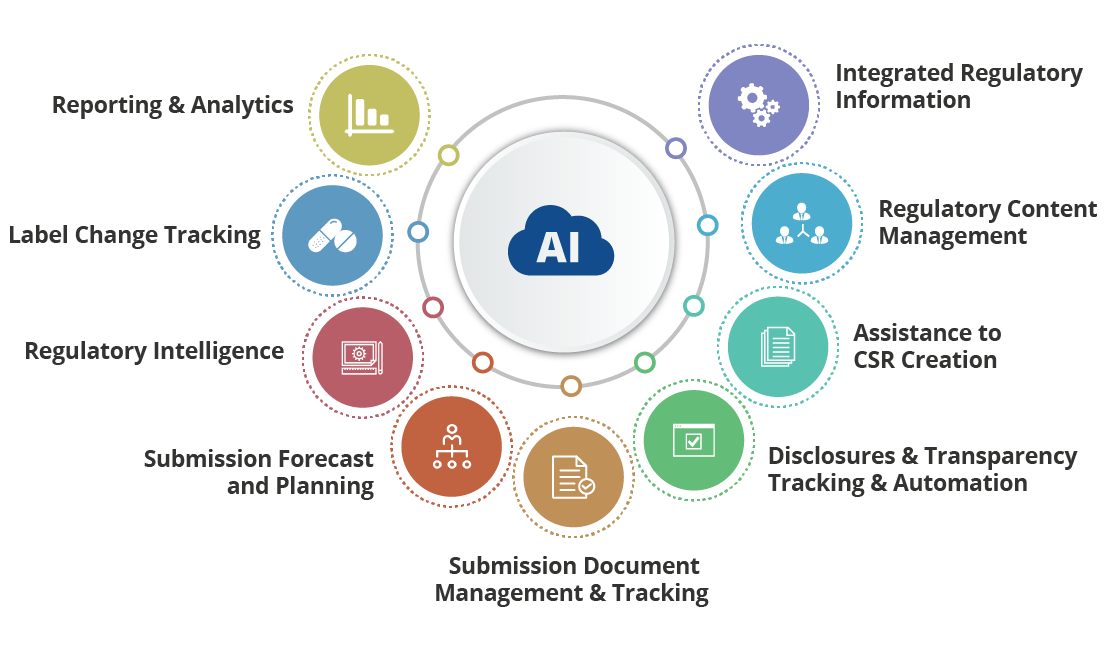
Addressing the concern, Regulatory Information Management – RIMTrack – is cloud-ready Artificial Intelligence (AI) based new age Regulatory Information Management System. It’s developed from scratch with the mission to help organizations to prepare submissions accurately and efficiently and by streamlining the regulatory processes related to tracking, licensing, approvals, regulatory and competitive intelligence, clinical trials, and reporting across global sites and stakeholders.
Designed to seamlessly integrate with various existing systems with data security at its core.
Enhanced
…………………………………………………………..
Collaboration | Data Quality | Tracking | Compliance Adherence | Consistency | Accountability
Reduced
…………………………………………………………
Costs | Submission Time | Complexity | Chaos
Get the most out of your Regulatory Information Management strategy

Maximize ROI
Integrate with existing RIM system and achieve complete end-to-end management of regulatory life cycle process.
Speed and Accuracy
Stay ahead of regulatory compliances by enhancing speed and accuracy of the submissions process.

Reduce OpEx
Eliminate or cut-down all your legacy systems and streamline entire regulatory information tracking.
Here is how RIMTrack helps you accelerate regulatory submission process
By replacing legacy systems built around old age tools and rigid applications with modern technologies and data processing & analytics tools and mobility, making it easy to collaborate, manage and track regulatory data easily and effectively.

Any Device
Workflow and Notification

Reporting

AI-driven document management

System Integration
It can also integrate with already existing RIM systems to provide an end-to-end and unified RIM solution.
Why efficient RIM system is needed?

A disciplined data-driven methodology will reduce complexity, streamline process and drive an efficient regulatory process

Small tier organizations are 20% more efficient than large organizations. Bigger the organization, larger the complexity

To establish the single, authoritative source of information and improve data quality

To achieve organizational flexibility and manage spikes in submission volume
Resources
Regulatory Information Management is an effective and easy-to-use solution that enhances collaboration and improves speed and accuracy of the regulatory submission process.
Bay Area Life sciences organization builds regulatory information management solution to reduce submission time.